BANNOCKBURN, IL — IPC released IPC-6012EM, Medical Applications Addendum to IPC-6012E, Qualification and Performance Specification for Rigid Printed Boards.
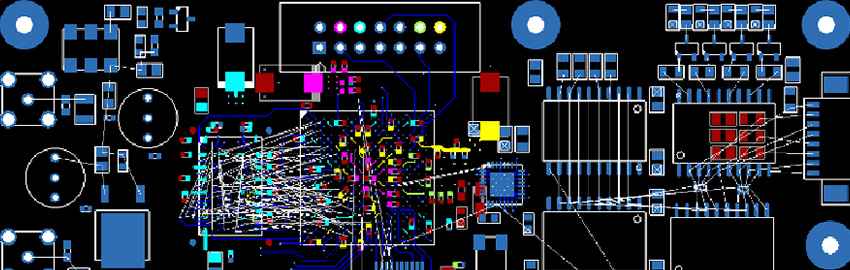
The IPC D-33AM Task Group developing IPC-6012EM realized there are two different focuses for electronics in the medical device industry sector: the high-volume production of standard-sized PCBs for medical diagnostic equipment applications and the miniature high-density PCBs for small devices, often human body implantable.
“We understand the medical industry utilizes electronics in laser surgical devices, radiation emitting devices, x-ray machines, ultrasound devices and implantables where product failure can result in the high risk of injury to the patient,” said John Perry, IPC director of printed board standards and technology. “IPC recognized the industry’s desire for more stringent printed board fabrication requirements than can be provided within the current IPC Class 3 Performance class for these types of medical devices. The IPC D-33AM Task Group was created to develop an addendum to the base IPC-6012E printed board performance specification that addresses those technological needs.”
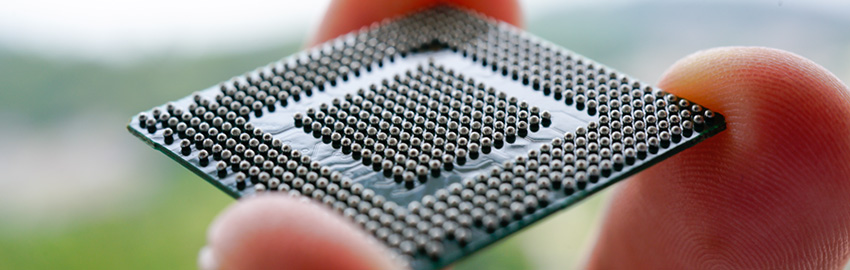
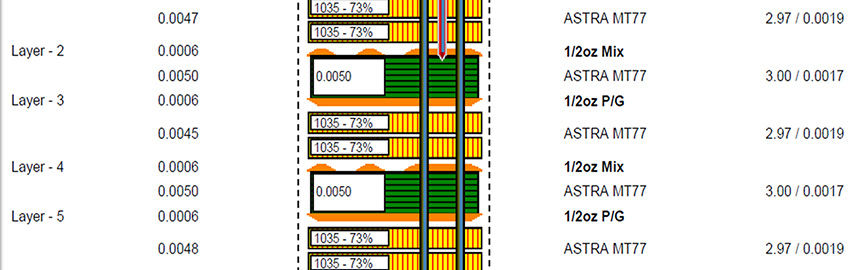
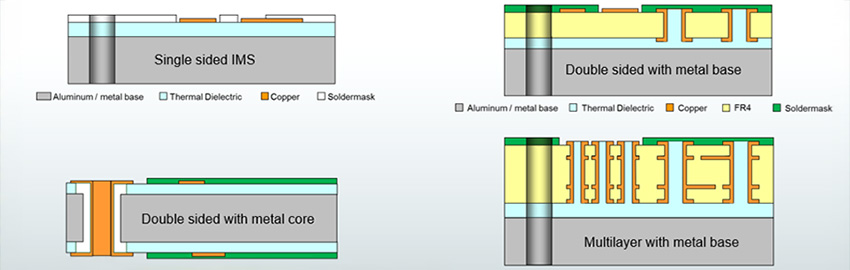
IPC-6012EM is the first addendum to an IPC specification that makes use of a new design level “D,” which was created to address the miniaturization level of medical devices. This new design level “D” goes beyond the typical feature sizes of what is typically considered HDI and addresses conductor width/spaces below 60µm, as well as via structures below 100µm.
Many regulatory requirements provided by both the US Food and Drug Administration and the European Union help ensure the safety and security of human beings and animals with respect to not only human and veterinary drugs and biological products, but also electronic medical devices. Examples include the EU Medical Device Directive, EU Active Implantable Medical Devices Directive and the EU Commission Regulations.
As noted by Andres Ojalill, IPC technical staff liaison to the IPC D-33AM Task Group, “IPC-6012EM has been written to streamline the production of high reliability printed boards for medical devices in accordance with regulations mentioned above so there are no gaps between technical and regulatory requirements.”
For more information, visit https://shop.ipc.org/IPC-6012EM-English-D.
PCB West Virtual 2020 has more than 125 hours’ worth of technical sessions on printed circuit design and manufacturing available through Oct. 12. pcbwest.com