Next-generation devices will rely on 3-D interconnect technology and power “scavenging.”
Ed.: This is the seventh of an occasional series by the authors of the 2017 iNEMI Roadmap. This information is excerpted from the Roadmap, which is available from iNEMI (http://community.inemi.org/content.asp?contentid=51).
Medical electronics research is moving into two camps: one consisting of traditional diagnostic, monitoring and implantable devices, and the second of cross-biological and silicon integration. Monitoring and implantable research will continue to be active areas of research. In these types of devices, the implantable or wearable contains a small radio. This radio will signal a cellular or other RF device, which in turn will collect data or forward it to the caregiver or medical personnel. Other types of connectivity include near-field communications (NFC), where two to four inches’ proximity between receiver and antenna enable the transference of data. These types of devices and connectivity require material research to find a material that is suitable for implantation and will still permit the radio signal to penetrate the body and the packaging material to reach the receiver. Good examples of these types of devices are the Proteus Digital Health pill monitor systems and a glucose-sensing contact lens from Google.
Power Innovations
There is always a need to enable longer life in implantable and wearable sensors, and researchers need to examine power delivery technology beyond traditional batteries such as scavenging and high-frequency inductive wireless powering. Power scavenging relies on the thermal energy of the patient’s body (up to 30μW/cm²), uses mechanical motion of the patient’s body (up to 10μW/cm²) or through-body wireless power transmission. Powering sensors in this manner extends the battery charging life and reduces the need for higher capacity batteries.
Alternatively, research into high-frequency power coupling (GHz range) as explored by the Poon research group at Stanford holds promise for dramatically smaller devices with ex vivo power sources. Biological and silicon integration is best explained as establishing the interconnection between the biologic device and the silicon. Examples:
- A dramatic demonstration of the promise of biological/electrical integration from the Kentucky Spinal Cord Injury Research Center shows a former college baseball player paralyzed below the waist regaining some functionality through use of an implantable neurostimulator.
- Neurons grown on silicon have been announced by the nonprofit Belgium research consortium IMEC. In this research, the neurons are grown on the silicon to make a device that enables the transistor to monitor brain activity and to sense the activity of a patient with epilepsy. Once the sensor detects the onset of a seizure, the silicon transistors counteract the brain’s erratic electrical impulses with specific trains of frequency, current and time, thereby curbing the severity of the seizure – or even repressing it alltogether.
These examples are the beginnings of the interconnection between the biological and electrical worlds. One can foresee the interaction of silicon devices with muscle tissue to reconstruct the neurological damage associated with spinal cord injury or muscular dystrophy. Continued focus in this research area is needed to make progress toward solving many of the crippling diseases that impact mankind.
Data Transfer
In the medical products group, there is no greater cry for development than the issue of total stack integration. No single infrastructure is established such that data may go seamlessly from any invivo sensor to recording device to patient records to health information exchanges back to the clinicians, laboratories, government entities, and even consumers themselves. Even the movement of patient records from one section of a hospital to another may cross two or more systems without a compatible means of moving the data seamlessly and securely.
Interoperability among medical devices continues to be a development challenge. With new standards, such as the Bluetooth Medical Device Profile specification or those from the Continua Alliance that create hardware and software building blocks, users will expect interoperability sooner rather than later – such as USB ports on computers or bank ATMs throughout the world – as demand increases in the home and consumer medical device market. Backend networking and analytics will be challenging, with different demands at single-point solutions. The “analytic continuum,” along with other seamless expectations (e.g., security, connectivity, service software) will demand interoperability occur during an individual’s day – through work, retail, health, hospitality, and automotive ecosystems – with a personalized user experience.
An important general trend in medical device technology is the need to continue miniaturizing devices using 3-D interconnect technology. This need is particularly acute in implantables, where smaller devices can help reduce procedural complexity and improve patient outcomes. There is an ongoing proliferation of use-cases for implantable medical devices where small size and power efficiency improvements are mandatory. One example is cardiac pacemakers, where intra-cardiac, lead-less architectures are being deployed. These pacemakers can be five to 10 times smaller than models deployed in 2012, with sizes, including battery, of less than 1cm3.
Another example is the bio-monitor (implantable or external). Certain procedures, such as cardiac ablations to correct cardiac arrhythmias, cost $50,000 to $75,000 per procedure. These need to be repeated in many instances due to low procedural yields. As bio-monitors become smaller and more capable, government or private insurers are likely to demand the use of biometric monitoring to assess patient burden and procedural effectiveness with objective data prior to authorizing initial or repeat procedures of this nature. This will be enabled by small, long lifetime biomonitors. For technology development teams, it will mean an accelerated demand for smaller and more capable connected monitoring devices that can operate 24/7 for years.
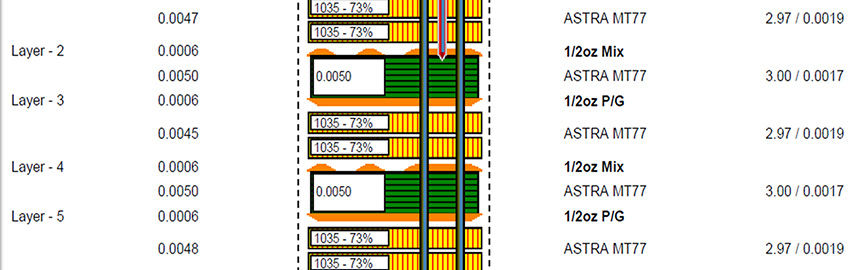
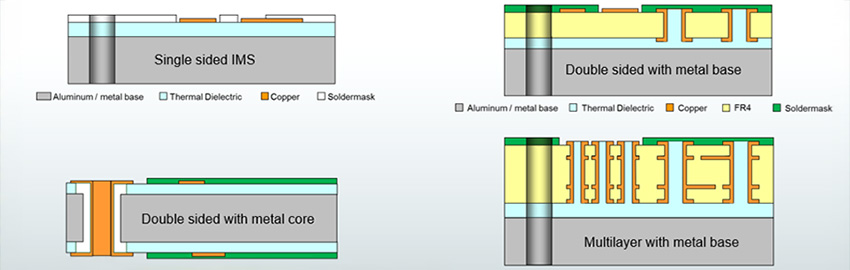
Realization of these devices will require 3-D SiP/heterogenous packaging of all system functions, including digital control, mixed signal ASICs, memory, sensors (magnetic field, 3-D motion, blood pressure, oxygenation, etc.) and RF telemetry (MICS or low-power Bluetooth). Similar trends exist for other active implantable devices, such as cochlear implants, neurostimulators (for pain management or deep brain stimulation) and bio-sensors.
‘Artificial Skin’ and Other Challenges
Among the technological challenges and trends facing medical electronics are:
- Stretchable and printed electronics. Stretchable electronics are a relatively new technology that will continue to find applications in the coming years. École Polytechnique Fédérale de Lausanne (EPFL) researchers recently announced conductive traces that can be bent and stretched up to four times their original length. They could be used in artificial skin, connected clothing or on-body sensors. Printed electronics has been introduced into medical technology but can still be considered embryonic for medical applications. Immediate products include pharmaceutical logistics, especially where temperature monitoring is concerned. MIT researchers reported on doped graphene combined with gold mesh to form a wearable patch for sweat-based diabetes monitoring and feedback therapy. The stretchable device consists of a heater, temperature, humidity, glucose and pH sensors and polymeric microneedles that can deliver drugs transcutaneously. Other researchers have demonstrated thin, flexible polymer light emitting diodes that act as sensors and displays.
- Medical device security. An increasing portion of medical devices are designed to be networked to facilitate care and therapy for patients and medical practitioners. Networked medical devices, like any networked computer system, are vulnerable to cyber-attack. In 2016 the FDA issued draft guidance, “Postmarket Management of Cybersecurity in Medical Devices.” The guidance was intended to inform industry and FDA staff of recommendations to address cybersecurity throughout the product lifecycle. The guidance suggests risk assessment, software validation and mitigation activities. Manufacturers may also implement hardware fixes to reduce certain device vulnerabilities. Device manufacturers must proactively address cybersecurity risks in medical devices to reduce the potential patient safety impact and overall risk to public health. iNEMI could play a role in helping to establish direction.
- Component and subassembly test methods. Many Class 2 medical and diagnostic system manufacturers use industry standard test methods. However, common standardized test methods for both components and second-level assemblies for implantables are still lacking. Vertically integrated manufacturers of pacemakers and ICDs have been successful using methods developed on their legacy devices. Standardized reliability test methods and reliability expectations for active implantable medical devices could increase component supplier diversity by providing clear and consistent requirements for participation in the implantable medical device market. Establishing minimum requirements for screening, such as burn-in, is needed. Such a standard would benefit existing manufacturers and new players.

An in-bore, real-time patient viewing system, allowing close patient monitoring and prospective motion correction for neurological MRI exams.
Donald Banks is a hardware design engineer for Abbott and chaired the Medical Product Emulator Group (PEG) of the 2017 Roadmap.