The mechanical aspects in a plating solution help to determine plating uniformity.
Over the past several columns, we focused on the chemical aspects of acid copper electrodeposition. It should not be inferred that chemistry is the only important parameter to be considered. Equal attention must be given to the mechanical aspects of plating, including solution agitation and solution filtration.
For uniform surface plating distribution, a homogeneous mixing of the electrolyte is necessary to avoid over plating of the surface while the through hole or blind via is being plated from the bottom up. The engineer should adjust flow rates so as not to create an excessively turbulent solution movement. Solution agitation of the copper-plating electrolyte may be accomplished with air agitation, eductors, solution impingement or cathode bar movement. The main purposes of agitation have been stated many times and include:
- Elimination of solution stagnation and dispersal of reaction products.
- Increase of deposition rates by mass transfer enhancement.
- Dissipation of heat at electrode/solution interfaces.
While the use of air agitation (supplied by a blower) was the standard method of agitating acid copper solutions for many years, circuit board designs have evolved to include more higher-density and smaller-diameter vias. Air agitation has reached its limitations. Air suffers from three main disadvantages. It has a chemical oxidative action towards solution constituents, it is electrically resistive when present as a cloud or foam of bubbles and the general plating rate enhancement is modest despite several possible parameters for adjustment. The least appreciated characteristic is the resistivity that can lead to an increase of electroplating voltage power of 25% to 30%, resulting in a significant electrical cost factor. It also generates environmental pollution through its dispersion of air bubbles. In addition, these tiny bubbles can lodge into the through holes and blind vias, leading to a reduction in plating thickness or voids.
One ideal solution to address this agitation need is to employ eductors to eliminate the use of air agitation completely. Eductors are solution jets based on the Venturi Principle, whereby one volume is pumped and up to four volumes are drawn in by the pressure drop, making it a highly efficient jetting system. When fully submerged, no air is entrained. Eductor agitation overcomes several of the disadvantages associated with air agitation while eliminating air bubbles and misting. In addition, eductor agitation provides a more uniform mixing of the plating solution, minimizing potential “dead spots” in the cell where air agitation is lacking. It is well known that educators provide more uniform agitation, better known as laminar flow. In contrast, air agitation provides a turbulent flow and may only promote mixing of the solution. For quality plating results, it is preferable to have interface agitation, where one interface agitation is directed more at the cathode diffusion layer. This helps to reduce the diffusion layer thickness, thus permitting the efficient delivery of additives and ions to the cathode surface.
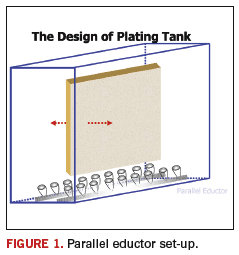
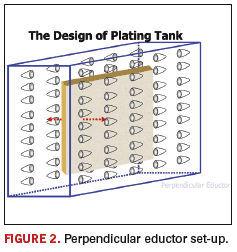
FIGURE 1 shows a schematic of a plating cell outfitted with eductors pointing up from the bottom of the cell. This is completely acceptable; however, there are other designs that have proven to perform equally as well. A schematic of one of those designs is shown in FIGURE 2.
Yes, chemistry is important to the success of any plating operation. However, mechanical aspects are just as critical. Spend some time setting up the most optimum filtration and agitation system possible, and you will be pleased with the quality of the plating finish and the overall plating distribution. PCD&F
Michael Carano is vice president for OM Group, Inc. and can be reached at This email address is being protected from spambots. You need JavaScript enabled to view it..