Influences on Barrier Performance of UV/EB Cured Polymers
A look at a number of variables reveals certain implications for materials used as moisture barriers for components.
Moisture vapor and oxygen transmission are both important considerations for many polymer applications, including sensitive electronics such as semiconductors and display components. The requirements for barriers range from extremely high to moderately permeable. Moisture vapor transmission rate (MVTR) is described as the rate of gaseous H2O passing through a film or container. Oxygen transmission rate, or OTR, is the rate of oxygen passing through a film or container. The standard measurements for both properties are similar. MVTR is measured in grams of H20/m2/day (gH20/m2∙day), and OTR is measured in cubic centimeters/meter2/day (cc/m2∙day). Both barrier transmission rates can be expressed based on the thickness of the film or container. This property is called permeation. As an example, 1 mil of a standard PET film will have an MVTR of roughly 25 grams of H2O passing through 1 m2 of PET in one day (25 gH2O/m2∙day). Because the transmission rate is inversely proportional to the thickness, 5 mils of PET will have an MVTR of 5 gH2O/m2∙day. A 1 mil thick film of PET will allow approximately 6 cc/m2∙day of oxygen. And a 5 mil thick PET film will allow 1.2 cc/m2∙day of oxygen to pass. In the English system of measurements, the permeability is defined at a thickness of 1 mil; therefore, the moisture vapor and oxygen permeability of PET would be 25 (gH2O ∙mil)/(m2∙day) and 6 (cc∙mil)/(m2∙day), respectively. By knowing the permeability of a material, the MVTR and OTR can be calculated at any thickness.
Different methods of measuring and reporting permeation can cause confusion. Relative humidity, temperature and dimensions of the test protocol can create large differences in the reported permeation values. For example, while PET may typically exhibit 25 (gH2O∙mil)/(m2∙day) at 37.8°C and 100% RH, this number drastically decreases at lower humidity levels and temperature. The same principles are considered when measuring OTR; most testing procedures use 23°C and 0% RH. However, some procedures require humidity or temperature alteration that will drastically change the MVTR and OTR. In addition, some industries may report permeability per cm2, or by 1mm thickness, or other variations. This PET could have a permeability of 6.35E10-5 if the value is reported in (gH2O∙mm)/(cm2∙day), and even lower at 90%RH and 25°C.
Moisture vapor transmission rate and permeability are important parameters for some polymer applications, and understanding how the values are reported is also important. The results shown eliminate the variable of thickness by reporting permeability for MVTR in g∙mil/m2∙day at 100% RH and 37.8°C (100°F). For OTR, data are reported in cc∙mil/m2∙day at 0% RH and 23°C.
Experimental
Sample preparation. Different neat oligomers and several blends were examined for MVTR, OTR and permeability. Each UV curable resin or blend was mixed with 4% photoinitiator (KTO46) and cured approximately 7 (±2) mils thick on polished aluminum, using two passes at 50 fpm under two mercury arc lamps (400W/in) in air. The samples were then removed from the aluminum and cut into the appropriate size for moisture vapor and oxygen transmission testing.
MVTR. The apparatus used for moisture vapor transmission testing was a Permatran W 3/33 from MOCON. The instrument parameters were 100% relative humidity, 37.8°C (100°F), and dry nitrogen flow rate of 100 sccm (standard cubic centimeters per minute). Samples were tested in duplicate and the average values taken for the result. The different samples were chosen to illustrate differences in permeability as a function of backbone chemistry, molecular weight, diluents of monomer, and crosslink density. A summary of the materials tested is shown in Table 1.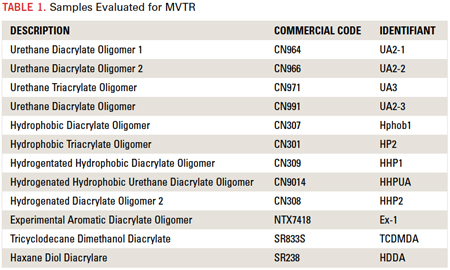
Samples were conditioned on the MOCON apparatus until equilibrium was achieved, typically for 12 to 24 hr. Equilibrium was defined as consistent results over a minimum 3 hr. time frame.
OTR. Many combinations of acrylate monomers and oligomers were sent to a third party to be tested for OTR. Samples were tested at 0% RH and 23°C. Oligomers and monomers ranging in functionality, aromatic/aliphatic urethane and epoxy backbones, and acid functional acrylates were all tested.
Calculations. Solubility parameters were calculated using the Hoy group contribution method, and were based on the idealized structure of the synthesized molecules. Unfortunately these are only calculations, and real experimental values would be more accurate for solubility parameters. Nonetheless, the author felt that these calculations provided some important insights into the nature of these results, so they were included.
Results and Discussion
MVTR. Several structural and chemical parameters had a notable influence on the moisture vapor permeability performance of the UV curable resins tested. The four primary factors contributing to the barrier performance of UV-cured polymers were 1) hydrophobic/hydrophilic nature, 2) molecular weight and crosslink density, 3) aromatic or cycloaliphatic nature, and 4) presence of unreactive fillers (solid particles).
The first influence readily identified as a major influence on permeability is hydrophobic/hydrophilic nature. CN307, CN308, CN309, CN310, CN9014 and CN301 are all on the extremely hydrophobic end of the scale for UV/EB curable oligomers. Materials like CN964, CN966J75, and CN971 are also hydrophobic in nature but much less so than the aforementioned structures. Without any regard to the other identified contributors, Figure 1 depicts hydrophobic nature (calculated Hildebrand Solubility parameters [δ]) vs. permeability for the oligomeric structures tested for this work. It is also interesting to note that some components of the Hildebrand parameter, in particular polarity (δp) and hydrogen bonding (δh), have more influence over water vapor transmission rates than dispersion (δd).
[Ed.: To enlarge the figure, right-click on it, then click View Image, then left-click on the figure.)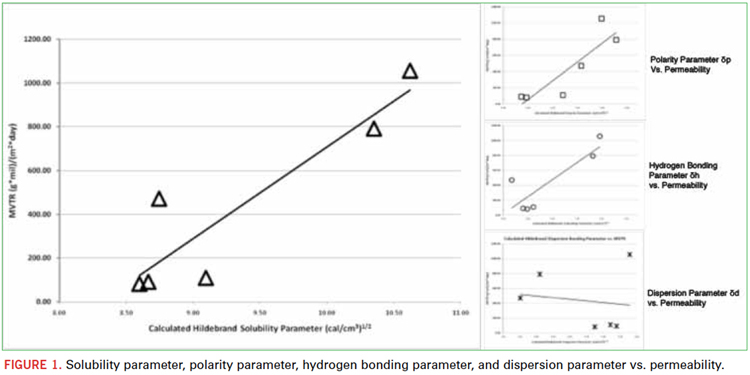
Clearly the implication is that more hydrophobic materials provide a better barrier to the ingress of water vapor. This is as expected, and the correlation to the calculated Hildebrand values serves to identify these calculations as a potential screening tool in the design of new or untested resins and commercial products. For the majority of materials tested in these experiments, degree of hydrophobicity was the dominant factor in barrier performance.
The second major influence on moisture barrier performance was molecular weight and crosslink density (Figure 2). Two oligomeric diacrylates with the same backbone chemistry, but different molecular weights, were tested. The lower molecular weight material exhibited much better barrier properties than the higher molecular weight oligomer. Backbone chemistry and the related degree of hydrophobicity had a much larger effect on barrier performance, but within the same backbone chemistry, lower molecular weight that yields shorter distance between acrylate-acrylate bonding exhibited barrier improvement. 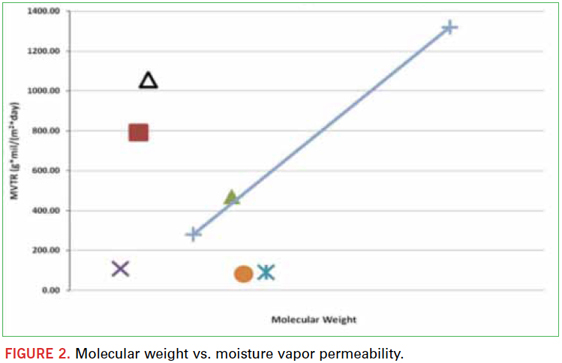
An easier way to evaluate the effect of crosslink density is to add varying concentrations of a low molecular weight diacrylate to increase crosslink density. In this experiment, hexanediol diacrylate (HDDA) was added at 20% by weight to some of the oligomers to observe this effect. Figure 3 shows that in all cases, the permeability decreased with the presence of HDDA, indicating that higher crosslink density and a tighter cured network improves barrier performance. In addition to HDDA, another monomer, tricyclodecane dimethanol diacrylate (TCDMDA) was added for comparison. The presence of TCDMDA, like HDDA, increased the crosslink density of the cured polymer and boosted the barrier performance. Both TCDMDA and HDDA are hydrophobic, but to a much lesser degree than the extremely hydrophobic oligomers; therefore, this effect can be attributed more to crosslink density and tighter network formation than to backbone chemistry and hydrophobicity.
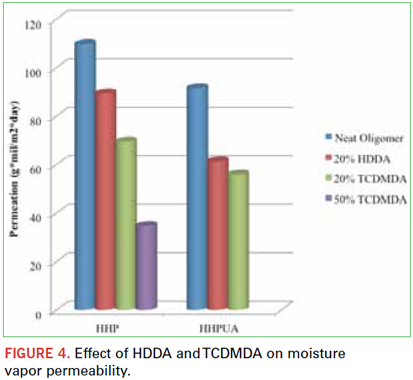
The next major contributor studied was the presence of aromatic and cycloaliphatic components in the cured polymer. It was shown that these two backbone chemistries improve barrier properties of similarly structured materials. A good example of this is the influence of HDDA vs. TCDMDA depicted in Figure 4. As discussed, lower molecular weight materials typically reduce permeability more effectively than higher molecular weight, but in this comparison, TCDMDA has a greater efficacy than HDDA, despite higher molecular weight (305 Daltons vs. 226 Daltons). Also interesting to note is that the number of carbons between acrylate groups for TCDMDA vs. HDDA is similar (7 vs. 6, respectively); therefore, the actual crosslink density is more similar than the molecular weight would suggest. Apparently the cycloaliphatic and more bulky backbone of TCDMDA better prevents ingress of H2O. At this point it should be noted that TCDMDA alone exhibited the best barrier performance: 17.8 (g∙mil)/(m2∙day) of any material tested in this experiment. Most likely this is due to the high crosslink density of the low molecular weight difunctional monomer and the overwhelmingly cycloaliphatic nature of its backbone. Aromatic backbones have a similar effect. A low molecular weight experimental product containing aromatic groups, NTX7418, exhibited low moisture vapor permeability of 69.5 (g∙mil)/(m2∙day).
The presence of unreactive fillers can influence the barrier properties of UV/EB cured polymers. Several clay materials were tested at different concentrations. In Figure 5, it was observed that, depending on type and concentration, unreactive fillers can be effective at reducing the moisture vapor permeability. Other more effective fillers certainly can be used, but this is outside the scope of this work. An entire paper devoted to the study of various fillers and loadings could easily be justified. Here, three different clay products were tested at 5%, 10%, 20%, and 30% loadings by weight. It should be noted that there was a dramatic effect on viscosity at 20% and 30% loadings. As viscosity becomes higher, free films are more difficult to cast without any imperfections that may adversely affect barrier performance. 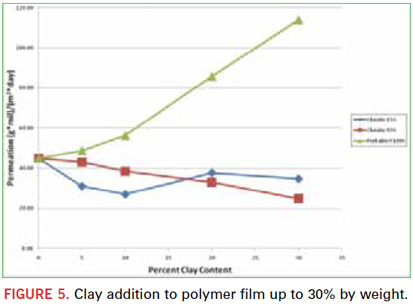
OTR. Many different monomer and oligomer structures were tested for OTR, but only a couple notable trends were observed. The samples displayed in Table 2 were neat monomers or oligomers prepared with 4% Esacure KTO46 photoinitiator and tested for OTR. In Table 3, blends of monomers and oligomers were evaluated using the same 4% photoinitator.
Figure 6 displays the oxygen permeation of both the neat and blended polymer films. It is evident that the hydrophobic/hydrophilic chemistries that lowered MVTR actually increased OTR. The CN309 and CN9014 blends are clearly much higher OTR than more polar molecules like CN146 and BCEA (Beta-carboxyethylacrylate). The low OTR is related to the concentration of carboxyl groups in the chemistry backbone. CN146 and BCEA contain carboxyl groups and yield a lower OTR
permeation value. The negative polarity of the carboxyl groups helps to block negatively charged O2 molecules from passing through the polymer film. An ideal polymer-based oxygen barrier film would contain a tight network of polar molecules with a maximized concentration of valence electrons. Neat BCEA has the lowest oxygen permeation due to the higher concentration of electron’s in the polymer network.
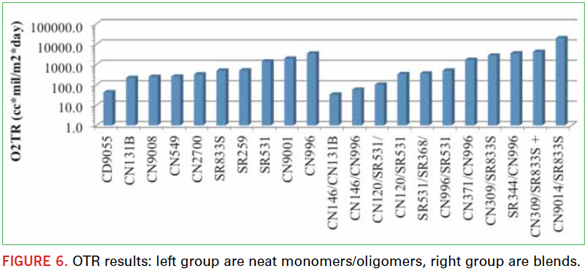
Aromatic and highly polar molecules will create a greater oxygen barrier due to their electron densities or presence of electrons. In Figure 6, an aromatic ring structure, CN146, is added to an aliphatic urethane acrylate (ALUA) CN996, and a significant reduction in oxygen permeation is observed. This is due to both the higher concentration of carboxyl groups and the aromatic backbone of CN146. Additionally, CN120 is a bisphenol A-based acrylate, containing aromatic rings in the backbone, and it also significantly reduces the oxygen ingress of CN996. When an aliphatic monomer SR344 is added to the same ALUA oligomer, there is only a slight reduction of oxygen permeation compared to the neat ALUA oligomer. It is evident that with higher electron density backbone components, for example aromatic rings and carboxyl groups, a moderately high oxygen barrier can be created using UV/EB cured monomers and oligomers.
An additional observation is that unreactive fillers, such as clay, may have a negative effect on oxygen resistance. With the addition of 10 parts clay added to a formulation, an increase of 1,430 cc∙mil/m2∙day was observed. This is a result of the reduction of polar forces or opening non-polar pores in the polymer film that allows greater ingress of oxygen. Other types of polar fillers, such as silica or metal, could be considered for future study.
Conclusion
The evaluation of the permeability of UV/EB cured polymers has highlighted several factors that influence performance. For MVTR, hydrophobicity, high crosslink density, and rich aromatic/cycloaliphatic content seem to be contributing factors for superior barrier performance. In addition, the use of unreactive fillers can provide an MVTR performance boost and an OTR drop. Although, this is not universal, and the types and concentration of fillers have not been exhaustively researched.
The results for OTR showed that it is important to use highly crosslinked aromatic and polar molecules to form a tight polymer network. A more concentrated grouping of highly polar and aromatic molecules will yield a better polymer oxygen barrier. For moderate to lower oxygen barrier properties, UV/EB cured polymer films are a viable option. For more demanding oxygen barriers, it is common practice to use polyvinylidene chloride (PVdc), polyvinyl alcohol (PVOH), or metallization layers. In the future of this study, it would be interesting to test fluroacrylate products for oxygen barriers, as other halogenated molecules demonstrate success.
Other aspects of the polymers listed need to be addressed. The best performing MVTR polymer in this study, TCDMDA, does not have all of the ideal polymeric behaviors that are needed for every application. TCDMDA is very brittle and hard, with a high tensile strength and Tg. While this could be ideal for some applications, for flexible packaging for example, the properties are not ideal. Due to the high crosslink density of TCDMDA, shrinkage amounts can be high, and coatings thicker than 3 to 5 mils may crack simply from the polymerization/curing reaction. Similarly, even though BCEA exhibits good oxygen permeability, because of the acid functionality, it would not be suitable for moisture exposure.
Joshua M. Oliver is manager of UV specialty applications, and Dale S. Babcock is applications chemist at Sartomer (sartomer.com); This email address is being protected from spambots. You need JavaScript enabled to view it..




