The Black and White of Immersion Tin
Published: 01 July 2009
by Allan Wilcox

Keeping an eye on cupric ions can eliminate black tin.
Every immersion tin plating operation faces the same problem during the immersion tin bath. Eventually, the bath will begin to plate black tin, and until recently, there has been no way to predict when the bath would begin to plate black tin. You might ask, “Why does this happen?”When immersion tin plates onto the copper, two atoms of copper dissolve for every atom of tin that plates out. The copper metal goes into solution as copper one (also known as cuprous ions, or Cu+). Copper in this form is well tolerated by most immersion tin plating solutions, and at reasonable levels, it has no effect. However, cuprous ions can react with atmospheric oxygen. When this happens, it is converted to copper two (cupric ions or Cu+2). This is the form of copper found in most electroplating baths, and it is blue in color. Cupric ions react with tin metal causing the copper to plate out. This copper plating out onto the tin is the black deposit that can appear on the top of the tin, often referred to as black tin.
Enter a new spectrophotometric method to measure cupric ions in any immersion tin solution. In theory, you could actually use your eye to estimate color as an indication of cupric ion build up, but a spectrophotometer will give you an exact reading.
To predict when the tin bath will plate black, measure the cupric level in the bath just when plating starts to darken and use this measure of Cu+2 in your tin bath to predict when it is time for a new bath. The maximum amount of cupric ion that your tin bath can tolerate is a function of specific bath chemistry, and it varies with different chemical suppliers. If an absolute standard is desired, 1000 ppm of copper can be achieved by adding 15 mls of standard copper sulfate solution to a liter of a fresh tin bath.
This measure should be done carefully, as cuprous ions in the bath sample can change rapidly to cupric ions; thus, conditions used by the method are designed to slow conversion of cuprous to cupric during the test. Some tin baths with an extremely high tolerance to cupric ions have been known to reach as high as 1400 ppm (1.4 grams/liter) of copper before the plating darkens.
Method Background
In order to understand the Zincon – Cupric Method, it is useful to discuss the science behind spectrophotometric methods. The critical detail is that spectrophotometric methods measure of the amount of a specific metal ion in a solution, based on the ability of a dye to form a complex with the metal ion of interest and causing a change in color of the dye.The Zincon-Cupric Method uses Zincon to selectively form a complex with the cupric ions. Whereas cupric ions form a stable, bright blue complex with Zincon, cuprous ions and tin ions do not form a complex with Zincon. The selectivity of Zincon for cupric ions and change in color is only part of what makes this method possible.
The amount of light absorbed by a solution is directly proportional to the amount of material in solution. So, as the amount of cupric ions increases in solutions of Zincon, more Zincon-Cupric complex is formed, and the solution color changes from orange-red to bright blue (FIGURE 1).
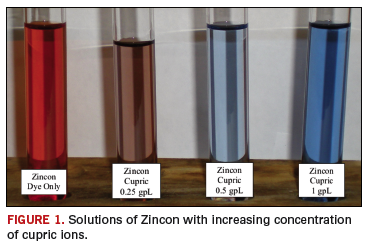
The properties of Zincon provide a method to measure how much cupric ion there is in an immersion tin solution. The method uses a spectrophotometer to measure how much light is absorbed at a specific wavelength of light.
Zincon-Cupric Method Specifics
The spectrum of uncomplexed Zincon in the indicator solution used in the Zincon-Cupric Method is shown in FIGURE 2 (red line). In the absence of cupric ion, the wavelength of maximum absorbance by Zincon is 490 nanometers (nm).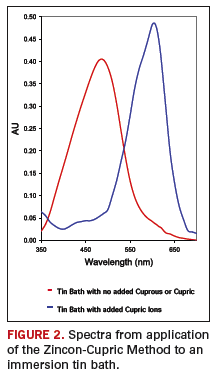
When an immersion tin solution is doped with cupric ions and the bath analyzed by the method, the complex formed between Zincon and cupric ions absorb most strongly at 605 nm (Figure 2, blue line).
The maximum allowable amount of cupric ion in an immersions tin solution can be measured and specified by the Zincon-Cupric Method. The method is very simple, consisting of adding a sample of the bath to an indicator solution, and measuring the absorbance at 605 nm with a spectrophotometer.
Procedure
1. Prepare the Zincon-Cupric Indicator Solution (1.0 Liter). To approximately 500 mls of deionized water add:100 mls Cupric Inhibitor*
100 mls Zincon Indicator concentrate**
2. Add a sample (1.0 mL) of the tin bath to the indicator solution.
3. Adjust pH to 10.4 with concentrated ammonia, then dilute to 1.0 liter with deionized water.
4. Allow solution to sit for 20 minutes for reaction to complete.
5. Read the absorbance at 605 nm by a spectrophotometer.
*The Cupric Inhibitor contains Thiourea (76 g/L) and Sodium Bisulfite (10 g/L) in deionized water.
**The Zincon Indicator Concentrate is 0.23 grams per liter Zincon indicator dissolved in 400 grams/liter Triethanolamine solution.
Note: For those attempting to make their own reagents, be aware. Conventional commercial Triethanolamine is actually 80% Triethanolamine + 20% Diethanolamine. The presence of Diethanolamine causes the Zincon indicator to become unstable. We are using a special grade of pure Triethanolamine available, commercially diluted to 85% with water.
Calculations
In the absolute sense, no calculations are necessary to monitor the build up of cupric ions with the Zincon-Cupric Method. Simply recording the absorbance at 605 nm is sufficient to establish the Zincon-Cupric Method for specific bath conditions. The maximum absorbance limit is obtained when the bath begins to plate out black tin.Conclusions
We have described a convenient method useful for measuring the amount of cupric in a tin bath. The method is very simple to implement and involves adding a sample of the tin bath to an indicator solution and then measuring the absorbance with a spectrophotometer. PCD&FAllan Wilcox is the technical service chemist with RD Chemical Company, Inc.; This email address is being protected from spambots. You need JavaScript enabled to view it..




