Improved Innerlayer Bonding for Sequential Lamination
Multiple lamination cycles add cumulative thermal stress to the innerlayer bonds.
Industry requirements to meet the demands of lead-free applications
have challenged many areas of PCB fabrication and design. These demands
have initiated a rapid influx of new dielectric materials, which,
despite increased thermal stress, must remain effectively bonded to
copper innerlayers. Delamination failures in advanced multilayer and
sequential-build products, caused by the conversion to lead-free
soldering, have also driven the need for improved oxide replacement
technology.
Such products must resist higher peak
temperatures, some 30°C greater than eutectic applications. Based on
statistically designed experiments, a new process has been developed
that delivers a more resilient copper conversion coating. The process
has shown improved bonding performance and greater stability at higher
temperatures, thus improving its capability for more thermal excursions
(assembly-reflow cycles at >260°C) without failure. This article
focuses on the product development criteria and performance evaluation
required to develop a process that is suitable for this challenging
environment. The testing and qualification will include data on peel
strength, solder float and IR-reflow testing.
Over
the past decade, traditional alkaline black oxide (or brown oxide)
bonding processes needed replacement due to a range of technical and
environmental issues as well as heightened cost pressures. The acidic
peroxide-sulfuric (oxide-replacement) processes quickly found favor due
to lower cost and more environmentally friendly chemistry. These
peroxide-sulfuric technologies, which use a primary organic additive to
enhance the surface texturing, were shown to deliver excellent bond
integrity in the pre-RoHS assembly setting.
However,
it comes as no surprise that continuing advances in PCB fabrication are
now pushing the performance requirements of these processes as well.
These include:
- Advanced board constructions / sequential lamination / microvia capable build-up processes
- New halogen free, high Tg and filled phenolic cured dielectric materials
- Demanding, high temperature lead-free assembly conditions
The Challenges
The
current peroxide-sulfuric oxide replacement process work based on a
“controlled etch” mechanism that typically removes 45 to 75 microinches
(1.1 to 1.9 μm) of copper. Innerlayers are first cleaned and then
conditioned in an alkaline medium, rinsed, and then are processed in
the peroxide-sulfuric micro-etching bath. The copper saturation
capacity of this bath is limited and has historically been on the order
of 18 to 22 g/L. Over the past three years, this technology has
improved, allowing a higher solution capacity of 50 g/L in high-volume
production environments. This capacity increase has been largely the
result of improved copper solubility coupled with greater process
stability.
The requirements for the high-yield
production of controlled impedance and fine line innerlayers also call
for further reductions in the copper etch levels. This low etch
approach greatly benefits high frequency signal integrity by
significantly reducing the “skin-effect” caused by a deep etch profile.
Reduced etching is often at odds with improved bond performance. The
need to improve bonding performance to meet the demands of new resin
systems and RoHS assembly temperatures, with minimal removal in the
order of 35 to 45 microinches (0.9 to 1.1 μm) of copper, has become a
benchmark for a “best in class” process.
Within
this challenge, higher values for peel strength and increased T260 and
T288 (time-to-delamination) values are continually sought, with the
process undergoing continuous review in order to deliver even greater
performance. This improvement is needed to meet the requirements
associated with the sequential lamination cycles applied in some
build-up processes, and to withstand the extreme thermal stresses
incurred in lead-free assembly and re-work.
The Oxide Replacement Process
The
peroxide-sulfuric oxide replacement process is controlled by a primary
organic material that modifies the etching mechanism to provide a
consistent, highly textured, and characteristic surface structure. Not
only does the primary organic additive accelerate the etch and modify
the copper surface, but it also chemically combines with the copper to
produce the characteristic organo-copper coating. This organic coating
is based mainly on cuprous (Cu+) oxide but also contains some cupric
(Cu+2) oxide. These give the conversion coating its characteristic
red-brown color. The schematic formation of the alternative oxide
coating is shown in Figure 1.
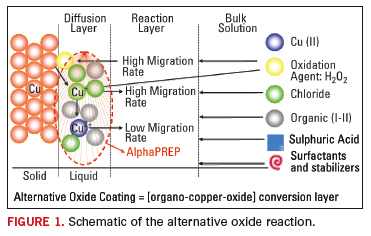
It
is well understood that a cuprous-rich oxide coating typically delivers
higher bond strength in the prepreg to copper layer. Cuprous oxide is
more thermally stable than cupric oxide. In addition, a cuprous rich
oxide has a higher chemical resistance to PTH chemistries, which can
give rise to “pink ring” through the attack and separation of the oxide
planes around the periphery of the holes. This chemical resistance is
critical not only for the metallization of high aspect ratio
through-holes, but more importantly for the landing pads on blind
microvias. It is possible to post treat the oxide conversion coating
using an alkaline process to remove the less resistant cupric oxide.
Such post treatment can increase the peel strength of the coating by 10
to 15%, but it does not improve the resistance to thermal delamination.
The elemental analysis is shown for both a standard and a post-treated coating. Figure 2
shows the actual XPS spectra of the as-produced coating and
post-treated coating (following a proprietary post dip). As would be
expected, the post-treated layers have a lighter more-reddish
appearance, indicative of the increased cuprous oxide content. This
coating composition is also outlined in Table 1.
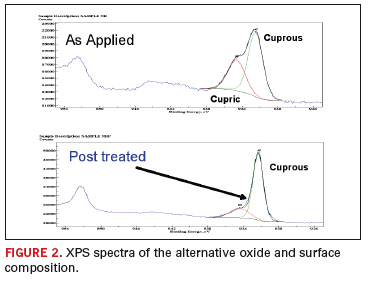
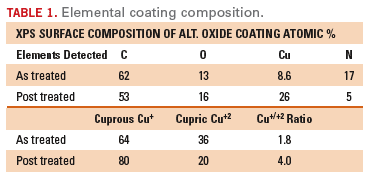
The Effect of Sequential Build
Increasingly,
PCB construction, especially for thin card mobile or PDA-type products,
is produced using sequential lamination techniques. Here, each
lamination cycle adds additional and cumulative thermal stress to the
innerlayer bond within the core construction. Depending on the
lamination temperature, (typically 190 to 200°C), this can
progressively reduce bond strength prior to final soldering, hence
increasing requirements for improved thermal integrity. The negative
impact on peel strength caused by sequential bonding cycles is shown in
Figure 3.
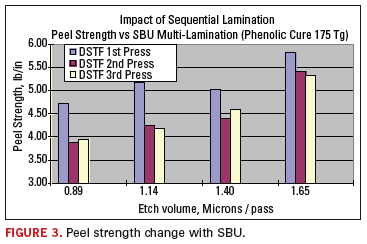
The
discussion of the repeated thermal cycles used in SBU fabrication must
include consideration of the added effects of the higher temperatures
seen in lead-free assembly. Exposure to multiple thermal cycles can
have a cumulative effect on bond integrity.
Impact of Lead-Free Assembly on the Alternative Oxide Bond
The
IR reflow soldering cycles used in assembly apply a large amount of
stress to both the core and sequential build layers. Although the peak
temperatures are targeted at 245 to 250°C, they can clearly overshoot
these values, potentially rising to 260°C or more. At this point, or
during the subsequent solder-wave application, the boards can fail due
to delamination. Large copper areas, especially those carrying very
high cluster-densities of small holes, can be especially vulnerable.
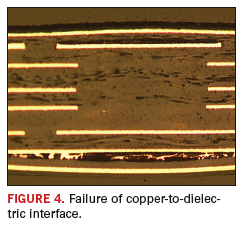
Delamination
can be triggered by a combination of many factors, and the first step
in determining root cause is to answer the key question – where is the
delamination occurring? The problem is typically diagnosed by using
cross section techniques to determine if the breakdown is between the
copper-to-dielectric layer (adhesive failure), or within the dielectric
itself (cohesive failure). Examples of these two conditions are shown
in Figure 4 and Figure 5, respectively.

Product Improvement Approach
Following
the transition to lead-free, the development group had already
completed work designed to provide alternative oxide products with
improved high temperature performance. An older, first generation, 25
g/L low copper capacity product (LCC) with improved thermal
stabilization was used as the benchmark. This LCC prototype product had
a demonstrated capability to withstand extended IR lead-free reflow
cycles, and was developed specifically for critical high-end
applications. For comparison, a 50 g/L high copper capacity (HCC)/low
etch product was used to address the requirements of higher signal
integrity, improved environmental capability (reduced waste), and lower
cost of ownership.
The HCC technology used organic
stabilization similar to the LCC product and had an already
demonstrated better performance for peel strength and thermal shock
resistance in high volume production environments. The defined approach
was to further optimize the HCC technology through more exhaustive
thermal stability testing, by using extensive L9 and L18 Taguchi
methodology.
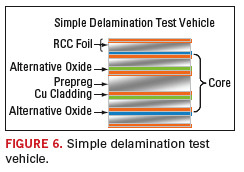
To test the product improvements against the benchmark, a rigorous testing protocol was developed. It included a test vehicle (Figure 6)
that was based on a six-layer board with a rigid FR-4 based four-layer
core. The FR-4 material was standard 150°C Tg epoxy. To simulate a
sequential build-up product, resin coated copper was added as
additional layers (one and six) to the four-layer core. Prior to
lamination of the resin coated copper foil, the core layers two and
five were electroplated with a standard acid copper process to simulate
a buried via construction. This build allowed the examination of the
effects of in-house electroplated copper on bond strength.
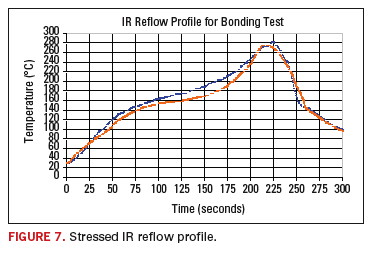
During
the testing process the test vehicles were subjected to high stress
thermal excursions of 270 to 280°C in an IR reflow chamber. The
failures that occurred could be found in either the central core or
within the surface layers. The profile of the IR reflow excursion is
shown in Figure 7.
Initial Benchmarking Fesults
Using
the IR profile described, copper innerlayers treated with the HCC
process were subjected to the reflow cycle. Where older generation
technology showed significant color change and pronounced thermal
breakdown effect at temperatures over 260°C, the HCC process was able
to withstand the peak of 270°C without any pronounced change of color.
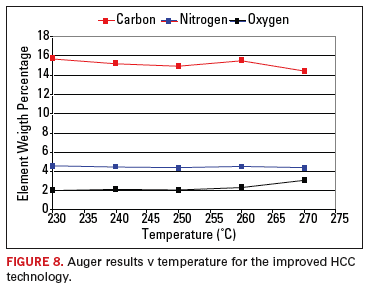
In
addition, Auger studies were used to determine the effects of
temperature on the organo-coating. The Auger tests showed no
significant loss (evaluating the elements Carbon and Nitrogen) or
oxidation of the coating. Further, significant improvements in
stability were seen over the lower soldering temperature range. No
perceptible change or evidence of oxidation was experienced. The Auger
results are shown in Figure 8.
The
improved HCC technology also demonstrated a 6 to 15% improvement in
peel strength of the coated layers. These results represent a major
step forward in improving the coating’s thermal resistance.
Furthermore, at 270°C the HCC technology was able to withstand an
average of 1.5 additional cycles before failure due to delamination
compared to the first generation processes.
Having
achieved very satisfactory improvement with the high copper capacity
formulation, the next step was to optimize the formula for thermal
performance using the Taguchi approaches previously described.
The
applied series of test arrays included a) the primary and secondary
inorganic acids (that influence the etching characteristic and
solubilize the copper); b) the organic components (that drive the
modified etch reaction rate, produce the organo conversion coating, and
stabilize the process and the coating); c) the grain refiner
(essentially a chloride based species), and finally; d) the oxidizing
agent – hydrogen peroxide (also drives the reaction-rate and texture
depth).
The studied responses were coating color,
etch-rate, pull/peel strength after coating, and most importantly, the
resistance to delamination using multiple IR reflow cycles with peak
temperatures of 270°C and 280°C respectively.
Details of some of the individual component plots, drawn from one of the later L18 arrays employed, are illustrated in Figure 9.
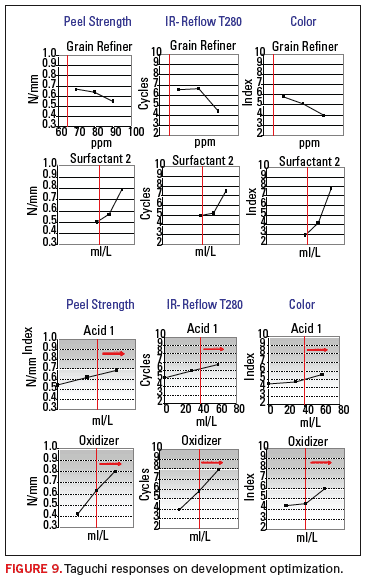
As
expected, varying levels of response, and some interactions, were seen
with many of the parameters employed. The findings were interesting for
several reasons. It was determined that only one organic additive
parameter significantly affected the color of the coating, but several
of the additives had a significant influence on the delamination
resistance. By contrast, the peel strength was mainly impacted by two
significant factors.
Interestingly, all the factors
that positively influenced the thermal performance also improved the
primary peel strength results. These factors also contributed to a good
aesthetic coating color and appearance. This can be seen in the
similarity of the displayed Taguchi responses that moved largely in
unison. This facilitated the completion of an optimized HCC product for
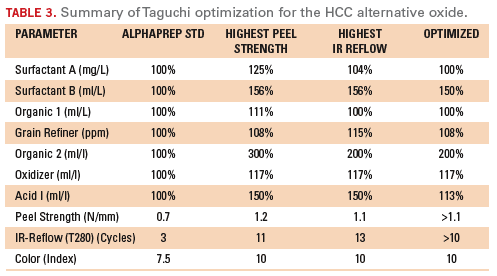
the confirmation runs. A summary of the Taguchi optimization is shown
in Table 3.
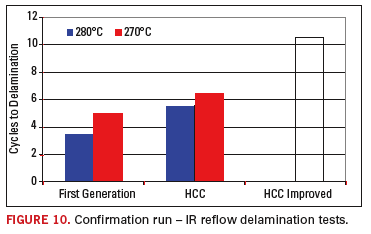
The
confirmation testing showed an expected and very positive gain with the
achievement of more than 10 IR reflow cycles without failure, based on
the aggressive test vehicle and profiles involved. The resulting
confirmation runs for delamination, as compared to the first generation
technology and HCC standard benchmarks are shown in Figure 10.
Conclusions
The
work described in this article has encompassed a long period of design,
development and testing, and was carried out over a three-year period.
Along the way, a lot of input has been received from the market, in
terms of both defining the challenge and applying best practices to
meet the growing industry requirement for improved PCB thermal
resilience.
Several leading PCB fabricators have
made significant progress in selecting improved materials and
incorporating better lamination and process procedures, all of which
open up the operating window for more demanding products. From this
work, it is clear that many factors can significantly and adversely
outweigh the alternative oxide contribution in achieving the balance
required for bullet-proof performance.
To play its
part, this study has been focused solely on finding ways to improve the
performance and contribution of the alternative oxide bonding
technology. The goal has been to provide a robust process with a wider
operating window offering better thermal resilience.
Conclusions
from this specific study can be summarized as follows. Delamination
failures due to lead-free thermal stress can be caused by many factors,
most significantly those involving the dielectric materials and the
lamination process. In the broader context, industry opinion is that
the alternative oxide process has a smaller impact than other material
factors in delamination. Improving the process can, however, make an
important contribution, especially for PCBs with large
copper-to-dielectric bonding areas such as internal ground planes.
This
can be seen with the older generation alternative oxide bonding
technology, which cannot consistently meet the increasing industry
requirement of highly advanced and performance sensitive designs for IR
reflows without blistering, delamination or related electrical failure
in lead free applications. The stability of the respective
organo-metallic conversion coating diminishes at 260°C, where bonding
performance decreases with each successive thermal excursion, leading
to potential delamination issues.
The use of
proprietary post treatments, which increase the cuprous oxide ratio can
provide improvements to peel strength, but do not necessarily solve the
thermal resilience challenge. One conclusion that can be drawn from
this work is that the industry-standard pull/peel strength measure
cannot adequately predict in-process bond strength, or a PCB’s
resistance to delamination under thermal stress.
Improved
LCC (low copper capacity) and HCC (high copper capacity) products have
already demonstrated higher thermal stability than their earlier
counterparts, along with greater capability to withstand multiple
lead-free reflows at inflated peak IR reflow temperatures of 270 to
280°C.
The laboratory work described in this article
establishes that an optimized HCC process can also more effectively
meet the 10 plus multiple reflow requirements associated with
sequential lamination and RoHS assembly simulation. Product design
specifications for a low-etch attribute (1.0 to 1.2 μm) for controlled
impedance, fine line integrity and improved high frequency signal
integrity have been key factors in this work. Maintaining the required
high copper capacity of 50 g/L delivers reduced environmental impact
and the lowest cost of ownership. PCD&F
Dr. Abayomi I. Owei is president/principal research scientist at Avo Tech International Inc.; This email address is being protected from spambots. You need JavaScript enabled to view it.. Dr. Jean Rasmussen is R&D manager, PWB metallization, at Enthone; This email address is being protected from spambots. You need JavaScript enabled to view it.. Dr. Axel Dombert is European technical manager, PWB products, at Enthone; This email address is being protected from spambots. You need JavaScript enabled to view it.. Danis Isik is a research scientist, PWB products, at Enthone; This email address is being protected from spambots. You need JavaScript enabled to view it.. David Ormerod is business director, PWB metallization, at Enthone; This email address is being protected from spambots. You need JavaScript enabled to view it..




